
6x HIS-TAGGED PROTEIN PURIFICATION
WITH PDC USING PDC KIT
BINDING CAPACITY IN SERIAL PROTEINS
OF NICKEL(II)-IMMOBILIZEDCHELATOR
FROM DIFFERENT COMPANY SUPPLIERS
Sample : human serum in excess
Matrix volume column : 1 ml of all Ni-Chelators and Cu-PDC-6FF except Qiagen 1.5 ml
Washing buffer : PBS 0.1 M pH 8.0
Elution buffer : PBS 50 mM, Imidazole 500mM pH 7.5
SDS PAGE 12% in the presence of b-mercaptoethanol
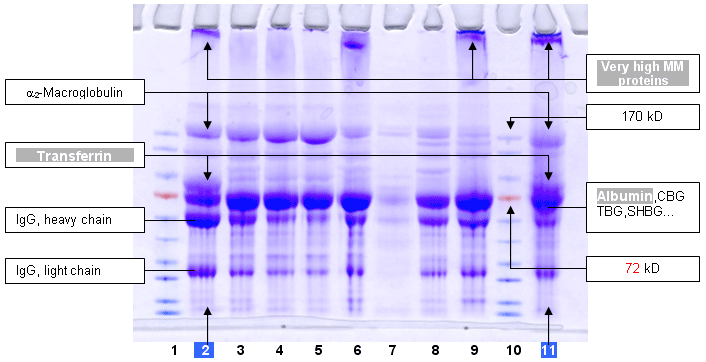
1, 10 : Markers expressed in kD : 170, 130, 95, 72, 55, 43, 34, 26, 17, 11
2 : Ni-Sepharose 6 fast flow from GE Healthcare
3 : Ni-PDC-Sepharose 6 fast flow (Ni-PDC-6FF) of Affiland
4 : Ni-PDC-Sepharose 4 fast flow (Ni-PDC-4FF) of Affiland
5 : Ni-PDC-Sepharose CL-4B (Ni-PDC-4S) of Affiland
6 : Ni-PDC-Sepharose CL-6B (Ni-PDC-6S) of Affiland
7 : Ni-His-link from Promega
8 : Ni-PDC-Streamline Quartz Base Matrix (Ni-PDC-SLQ) of Affiland
9 : Ni-NTA from Qiagen
11 : Cu-PDC-Sepharose 6 fast flow (Cu-PDC-6FF) of Affiland
Binding capacity in serial proteins (lyophilized, free of salt)
expressed in milligrams per ml of resin
Affiland Ni-PDC-6FF : 15 mg/ml resin |
Affiland Ni-PDC-4FF : 12 mg/ml resin |
Affiland Ni-PDC-4S : 12 mg/ml resin |
Affiland Ni-PDC-6S : 18 mg/ml resin |
Affiland Ni-PDC-SLQ : 10 mg/ml resin |
Affiland Cu-PDC-6FF > 50 mg/ ml resin |
PDCR : pentadentate chelator, patented products of Affiland |
|
GE Healthcare Ni-6 fast flow : 12 mg/ml resin |
Qiagen Ni-NTA : 8 mg/ml resin |
|
Promega His-link : approx.1 mg/ml resin |
|
OBSERVATION : GE Healthcare Ni-6 fast flow gives the same qualitative results as |
|
Most 6x His-tagged protein purifications are performed with Metal chelate resins: some with Ni-NTA and others with Co chelated resins.
However, it is known now that
- Some 6x His-tagged proteins do not bind at all to Ni-NTA or Co-NTA resins. This is due to the non-accessibility of the polyhistidine tag.
- The 6x His-tagged proteins from E. Coli expression systems, purified by Ni-NTA resin for example, generally are contaminated by proteins of bacterial origin or by metal binding proteases.
- Our PDC KIT consisting of four Metal-Pentadentate chelator-matrix (M-PDC): Cu-PDC, Ni-PDC, Zn-PDC & Co-PDC ready-to-use prepacked in polypropylene columns, overcomes the problems cited above.
- The selection of the most appropriate metal chelate column in a primary screening.
- Then , the optimization of the conditions for a single step purification.
- And finally, the scale-up of the purification, if necessary.
APPLICATIONS
Example 1 : with PDC-Sepharose CL-4B Kit (PDC-4S KIT)
The crude clarified lysate of 6x His-tagged HSP 60 from Helicobacter pylori expressed in E. Coli was kindly supplied by E. Baise, Laboratoire de Biochimie B6, Sart Tilman 4000 Liège (Belgium).
Sample volume that was loaded onto each column: 500 µl (conc. in HSP 60 : approx. 5 mg/ml).
SDS-PAGE 12% in the presence of beta-mercaptoethanol
(15 µl of sample - stained with Coomassie blue)
Panel A (Cu-PDC)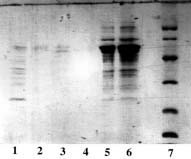 |
Panel B (Ni-PDC)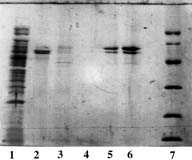 |
|---|---|
Panel C (Zn-PDC)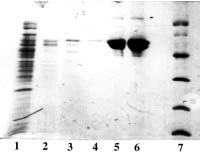 |
Panel D (Co-PDC)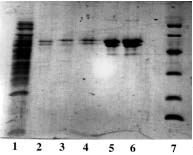 |
|
Panel A, B, C & D Lane 1: flow through + buffer A; Lane 2: buffer A; Lane 3: buffer B; Lane 4: buffer C; Lane 5,6: buffer E; Lane 7: Markers (97,400; 66,200; 45,000; 31,000; 21,500 & 14,400 daltons). Conclusion: Zn-PDC allowed the purification of HSP 60 in a single step with a recovery of 15 mg of protein per ml of wet gel (Panel C, lane 5 & 6). |
|
Example 2 : with PDC-Sepharose CL-4B Kit (PDC-4S KIT)
The crude clarified lysate of 6x His-tagged Urease from Helicobacter pylori expressed in E. Coli was kindly supplied by J.M. François, Laboratoire de Biochimie B6, Sart Tilman 4000 Liège (Belgium).
Sample volume that was loaded onto each column: 2 ml (conc. in urease: approx. 1 mg/ml).
SDS-PAGE 12% in the presence of beta-mercaptoethanol
(15 µl of sample - stained with Coomassie blue)
Panel A (Cu-PDC)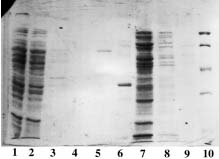 |
Panel B (Ni-PDC)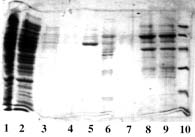 |
|---|---|
Panel C (Zn-PDC)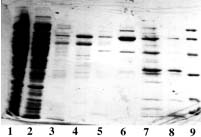 |
Panel D (Co-PDC)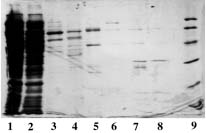 |
|
Panel A Lane 1: flow through; Lane 2,3,4: buffer A; Lane 5: buffer B; Lane 6: buffer C; Lane 7,8: buffer E; Lane 9: buffer F and Lane 10: Markers (97,400; 66,200; 45,000; 31,000; 21,500 & 14,400 daltons). Panel B Lane 1: flow through; Lane 2,3,4: buffer A; Lane 5: buffer B; Lane 6: buffer C, Lane 7: buffer D; Lane 8,9: buffer E and Lane 10: Markers (97,400; 66,200; 45,000; 31,000; 21,500 & 14,400 daltons). Panel C & D Lane 1: flow through; Lane 2,3: buffer A; Lane 4: buffer B; Lane 5: buffer C; Lane 6: buffer D; Lane 7,8: buffer E and Lane 9: Markers (97,400; 66,200; 45,000; 31,000; 21,500 & 14,400 daltons). Conclusions: Ni-PDC can be used to purify the native urease (Panel B, lane 8 & 9) and Zn-PDC to obtain the alpha-chain (MW 60,000 daltons) and the beta-chain (MW 30,000 daltons) of urease (Panel C, lane 6 and 7 & 8). |
|
Example 3 : with PDC-Sepharose CL-4B Kit (PDC-4S KIT)
The crude clarified lysate of 6x His-tagged Penicilin binding protein 5 MW=70,000 daltons, expressed in E. Coli was kindly supplied by P. Partoune, Centre d'Ingénierie des Protéines, Institut de Chimie B6, Sart Tilman 4000 Liège (Belgium).
Sample volume that was loaded onto each column: 2 ml (conc. in PBP5: approx. 0.1 mg/ml).
SDS-PAGE 12% in the presence of beta-mercaptoethanol
(15 µl of sample - stained with Coomassie blue)
Panel A (Cu-PDC)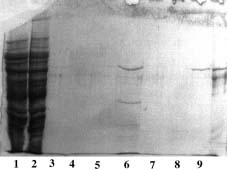 |
Panel B (Ni-PDC)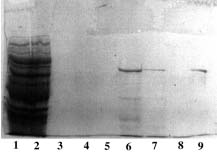 |
|---|---|
Panel C (Zn-PDC)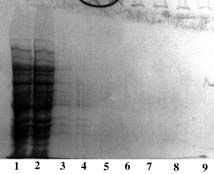 |
Panel D (Co-PDC)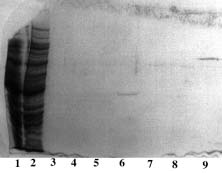 |
|
Panel A, B, C & D Lane 1: crude supernatant; Lane 2: flow through + buffer A; Lane 3, 4: buffer B; Lane 5,6,7: buffer E; Lane 8: buffer F; Lane 9: reference (pure product MW 70,000 daltons). Conclusion: Ni-PDC is the best for this purification (Panel B, lane 6 & 7). |
|
Example 4 : with PDC-Sepharose Fast Flow Kit (PDC-FF KIT) New!
FIRST ATTEMPTS
at
6xhis-r-BlaZ purification with PDC-6FF KIT
(6FF = Sepharose 6 Fast Flow)
Sample : 6xhis-r-BlaZ crude extract. The flow through was reloaded 3 times
Matrix volume : 1 ml Cu-PDC-6FF, Zn-PDC-6FF, Ni-PDC-6FF, Co-PDC-6FF column
Washing buffer : buffer A = PBS 0.1 M pH 8.0 and
buffer B = PBS 0.1 M, urea 4M pH 8.0
Elution buffer : buffer E = PBS 50 mM, Imidazole 105 mM pH 7.5,
buffer F = PBS 50 mM, Imidazole 300mM pH 7.5 and
buffer G = PBS 50 mM, Imidazole 500mM pH 7.5
SDS PAGE 12% in the presence of beta-mercaptoethanol
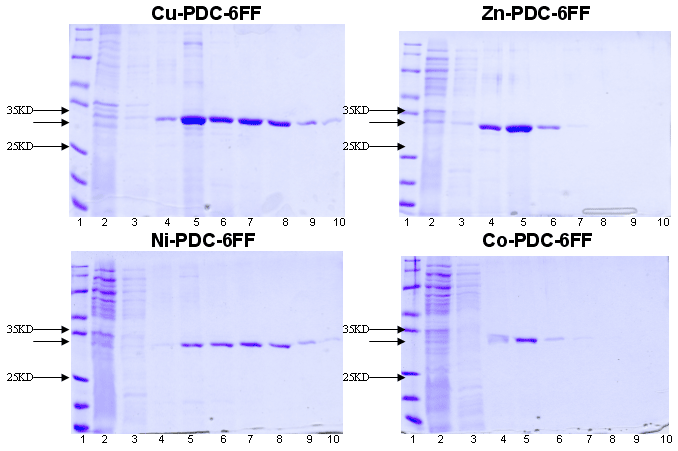
Lane 1 : Markers expressed in KD : 116.0, 62.0, 45.0, 35.0, 25.0, 18.4, 14.4 |
Lane 5 : buffer E, fraction 1 |
Conclusion : All Cu, Zn, Ni and Co-PDC-SLQ may be candidates for the purification of this 6xhis-r-BlaZ.
Example 5 : with PDC-Sepharose Fast Flow Kit (PDC-FF KIT) New!
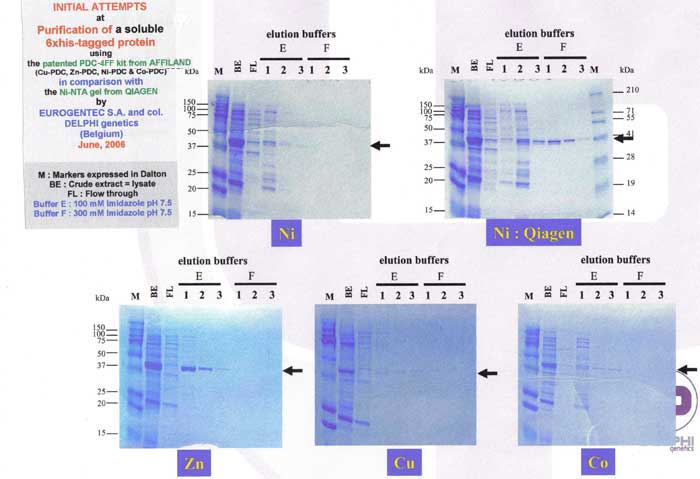
Example 6 : with PDC Streamline Quartz Base Matrix (PDC-SLQ KIT) New!
INITIAL ATTEMPTS
at
6xhis-r-BlaZ purification with PDC-SLQ KIT
(SLQ = Streamline Quartz Base Matrix)
Sample : 6xhis-r-BlaZ crude extract. The flow through was reloaded 3 times.
Matrix volume 1 ml Cu-PDC-SLQ, Zn-PDC-SLQ, Ni-PDC-SLQ, Co-PDC-SLQ column
Washing buffer buffer A = PBS 0.1 M pH 8.0 and
buffer B = PBS 0.1 M, urea 4M pH 8.0
Elution buffer : buffer E = PBS 50 mM, Imidazole 125 mM pH 7.5,
buffer F = PBS 50 mM, Imidazole 300mM pH 7.5 and
buffer G = PBS 50 mM, Imidazole 500mM pH 7.5
SDS PAGE 12% in the presence of beta-mercaptoethanol
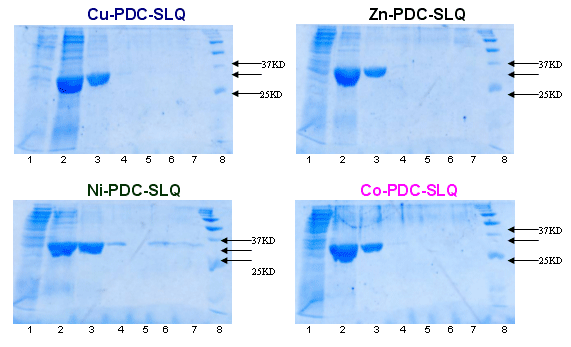
Lane 1 : flow through
Lane 2 : buffer E, fraction 1
Lane 3 : buffer E, fraction 2
Lane 4 : buffer F, fraction 1
Lane 5 : buffer F, fraction 2
Lane 6 : buffer G, fraction 1
Lane 7 : buffer G, fraction 2
Lane 8 : Markers expressed in KD : 250, 150, 100, 75, 50, 37, 25, 15, 10
Conclusion : All Cu, Zn, Ni and Co-PDC-SLQ can be candidates for the purification of this 6xhis-r-BlaZ.
Example 7 : with PDC Streamline Quartz Base Matrix (PDC-SLQ KIT) New!
INITIAL ATTEMPTS
at
6xhis-r-Propeptide purification with PDC-SLQ KIT
(SLQ = StreamlineTM Quartz Base Matrix)
Sample : 6xhis-r-Propeptide crude extract. The flow through was 3 times reloaded
Matrix volume : 1 ml Cu-PDC-SLQ, Zn-PDC-SLQ, Ni-PDC-SLQ, Co-PDC-SLQ
Washing buffer : buffer A = PBS 0.1M pH 8 and
buffer B = PBS 0.1M, urea 4M pH 8.0
Elution buffer : buffer E = PBS 50 mM, Imidazole 105 mM pH 7.5
buffer F = PBS 50 mM, Imidazole 300mM pH 7.5
SDS PAGE 15% in the presence of beta-mercaptoethanol
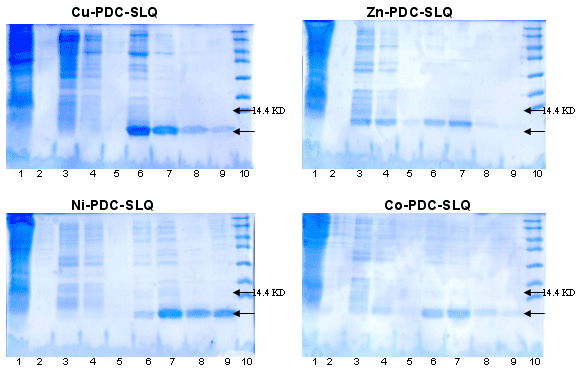
Lane 1 : flow through Lane 2 : Washing buffer A,10th fraction of 4ml |
Lane 6 : Elution buffer E, fraction 1 of 2ml |
Conclusion : Zn-PDC-SLQ is recommended for scaling up the purification of this 6xhis-r-propeptide.
Example 8 : with PDC Streamline Quartz Base Matrix (PDC-SLQ KIT) New!
FIRST ATTEMPTS
at
6xhis-r-IGF2 purification with PDC-SLQ KIT
(SLQ = StreamlineTM Quartz Base Matrix)
Sample : 6xhis-r-IGF 2 crude extract. The flow though was 3 times reloaded.
Matrix volume : 1 ml Cu-PDC-SLQ, Zn-PDC-SLQ, Ni-PDC-SLQ, Co-PDC-SLQ
Washing buffer : buffer A = PBS 0.1M pH 8.0
buffer B = PBS 0.1 M, urea 4M pH 8.0
Elution buffer : buffer E = PBS 50 mM, Imidazole 125 mM pH 7.5
buffer F = PBS 50 mM, Imidazole 300mM pH 7.5 and
buffer G = PBS 50 mM, Imidazole 500mM pH 7.5
SDS PAGE 15% in the presence of b-mercaptoethanol
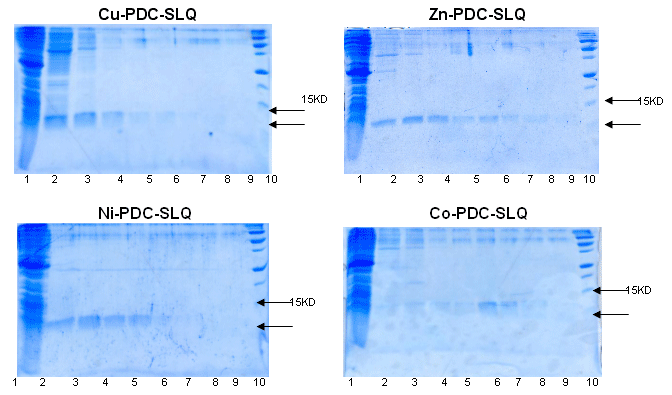
Lane 1 : flow through |
Lane 6 : buffer F, fraction 1 |
Conclusion : The Zn-PDC-SLQ and Ni-PDC-SLQ can be used for scaling-up the purification of this 6xhis-r-IGF-2
PROTOCOL FOR 6x HIS-TAGGED PROTEIN PURIFICATION WITH PDC-MATRIX
1. Selection of the most appropriate Metal chelate column in a primary screening with PDC KIT
- Equilibrate 4 columns of 1 ml of Cu-PDC, Ni-PDC, Zn-PDC and Co-PDC with 4 ml of buffer A.
- Load 0.5 ml-10 ml of clarified lysate* to each column. Recycle 3 times.
(It is recommended to use PBS pH 8.0 in any case, as the loading buffer). - Wash each column 3 times with 4 ml of buffer A.
- Wash each column 3 times with 4 ml of buffer B.
- Wash each column once with 4 ml of buffer A.
- Elute each column 4 times with 2 ml of buffer E.
- Elute each column 4 times with 2 ml of buffer F.
- Assay all the fractions obtained from point 2 to point 7 using the most appropriate system (O.D at 280nm, SDS-PAGE etc.).
- non-denaturating conditions, with a sonication buffer such as PBS 50mM pH 8.0 with or without EDTA 0.5M (0.1 - 0.2% v/v).
PBS: phosphate buffered saline (Na2HPO4 50mM-100mM, NaCl 150mM-1000mM pH 8.0. - or denaturating conditions with a lysis buffer such as PBS containing guanidine hydrochloride 6M or urea 8M pH 8.0 for example, with or without beta-mercaptoethanol (approx. 1% v/v). In this case, the buffer A (step 1) can be replaced by PBS buffer containing guanidine hydrochloride 6M pH 8.0 and the steps 4, 6, 7 and 8 are not yet necessary. The buffers E & F can contain beta-mercaptoethanol.
2. Optimization of a single step purification of 6x His-tagged proteins with PDC-Matrix
Once the most appropriate column is selected, the optimization of the chromatography conditions can be performed as follows.
The following remarks should be taken into account:
- The use of hydrogenocarbonate or carbonate buffer as loading and washing buffer is not recommended, because of their strong competing electron donor;
- The Tris.HCl or Tris.AcOH pH 8.0 buffer can be used as loading and washing buffer when the affinity of metal ion-PDC complex for the protein of interest is fairly high;
- The phosphate 20mM-100mM pH 8.0 buffer is often used as loading and washing buffer;
- A high concentration of NaCl (0.3M-1.5M) in the washing buffer, generally does not affect the binding capacity of the metal chelate gel and the activity of the protein of interest . The role of NaCl is to dissociate interactions between protein-protein or generally to avoid any ion-exchange effects;
- The use of guanidine hydrochloride 4M-6M pH 8.0 in the washing steps, generally does not affect the activity of any proteins which are not eluted by this buffer. Note that the guanidine hydrochloride 4M-6M pH 8.0 buffer tends to strip slowly the Cu-Chelator column if excessively used;
- The urea 2M-8M pH 8.0 generally does not affect the binding capacity of the Metal chelate-PDC for 6x His-tagged proteins. In particular, the proteins eluted in urea over a pH range of 6.0-8.0 generally remain biologically stable;
- The use of NaH2PO4 50mM-100mM, NaCl 150mM-300mM pH 6.0 tends to eliminate some proteins having affinity lower than 6x His-tagged proteins;
- The washing buffer pH 8.0 containing imidazole 05mM-20mM, can be used to reduce the non-specific binding;
- The elution can be realized with a gradient of imidazole 20mM - 300mM pH 7.4 or a gradient of NaH2PO4 50mM pH 7.4 - 4.0.
Important note
- It is not recommended to use regenerated columns for the initial protein screening. New columns should be used.
- But the regenerated gel can be used for purification of proteins in the optimization step.